Researchers identify which cancer cell subpopulations cause tumor growth and metastasis
Researchers from the VIB-KU Leuven Center for Cancer Biology have discovered which cancer subpopulations are responsible for the growth of tumors and subsequent metastasis in melanoma using cutting-edge technology at single cell resolution. A paper published in Nature by Jean-Christophe Marine and his colleagues from the Marine Lab shows for the first time, that the proximity of endothelial cells acts as a catalyst for cancer cells in their ability to stimulate tumor growth. Moreover, using spatial imaging technologies, the team was able to identify a rare type of cancer cells that lies at the basis of metastatic dissemination, providing new targets for the prevention of metastasis.
While cancer research has come a long way over the past years and patient survival has improved dramatically thanks to novel therapeutic options such as immune therapy, radiotherapy and improved surgery, there remain many challenges in the fight against cancer. Specifically in the case of melanoma, one of the most aggressive forms of skin cancer, early detection is essential for treatment. With more than 150,000 diagnoses accounting for 4% of all new cancer cases in 2020, it is the 6th most common cancer in Europe and is becoming more prevalent each year. Thanks to the findings of Jean-Christophe Marine and his team at the VIB-KU Leuven Center for Cancer Biology, however, new methods for early detection and treatment might be on the horizon.
Using specialized cutting-edge technology, the Marine lab was able to study melanoma tumors at the individual cell level effectively mapping out the cellular composition of a melanoma tumor both in time and space (3-dimensional context of tumor). In doing so, the team established that melanoma growth is hierarchically organized with some cancer cells fueling tumor expansion, while others did not.
Jean-Christophe Marine, Center for Cancer Biology: “Using transformative technologies, we were able to dissect the composition of tumors at an unprecedented level of resolution. This has enabled us to identify a wide variety of distinct cancer cell populations as well as normal cell types infiltrating these tumors. Importantly, we found that the proximity to endothelial cells causes cancer cells to acquire the capacity to fuel tumors. A stunning discovery, because it suggests that blocking communication between these two cell types could prove to be an attractive avenue to prevent tumor growth at an early stage.”
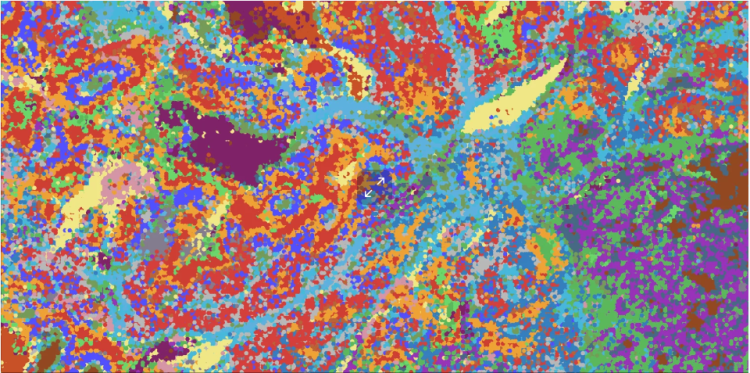
Spatial distribution of differen melanoma cell populations in their microenvironment.
Origins of metastasis unraveled
Melanoma tumors contain millions of cells, but not all of these have the same function. In addition to insights on tumor growth, the Marine Lab also identified a rare cell type making up only 2% of a tumor that does not contribute to tumor growth. Instead, this specific cell type is at the basis of the process of metastatic dissemination, the spreading of melanoma cells to distant vital organs.
By generating new sophisticated mouse models to trace melanoma cells, dubbed Met-Track, the team was able to unravel the identity and spatial distribution of each cell within a given melanoma tumor.
Jean-Christophe Marine: “Once we started tracking specific melanoma cell types in our refined mouse model, we found that only a small fraction of cells, which exhibited invasive characteristics and were localized deep in the tumor tissue, was alone responsible for the seeding of all metastatic lesions in distant organs. Once these cells colonize distant organs such as the lungs or liver, they switch identities and start proliferating in their newfound locations, a process that severely compromises patient survival. These data are important as they show that the presence of such cells in primary melanoma lesions is a key indicator to predict if there is a risk of metastasis.”
The recent discoveries of the Marine Lab further establish the important role of the tumor microenvironment in shaping tumor evolution and offer a platform for the identification of targetable vulnerabilities in the tumor. Additionally, these findings could help in predicting the aggressiveness of a tumor early on, potentially leading to new strategies that can help delay or even prevent tumor growth.

Jean-Christophe Marine and Panagiotis Karras of the Center for Cancer Biology
