Fruit flies provide insights into the hidden world of sleep
We spend around one-third of our lives sleeping, yet scientists still don't entirely understand how sleep works and why it's so important. Published in Nature Neuroscience, researchers from the Fly Sleep Lab of Prof. Sha Liu at the VIB-KU Leuven Center for Brain & Disease Research use single-cell technology to define sleep at the smallest unit possible – the expression of individual genes. Their discoveries pave the way to unraveling sleep's underlying biological mechanisms.
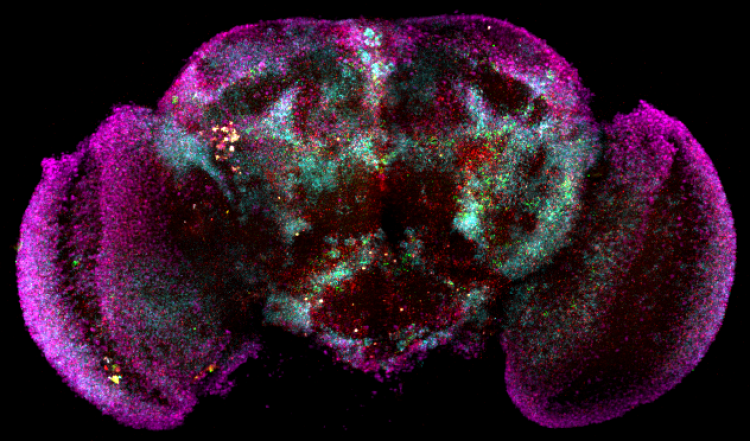
mRNA expression in the fly brain
Like drinking water and eating nutritious food, getting a regular good night's sleep is important for a healthy and functioning body and mind. Despite the clear importance of sleep, scientists still don't entirely understand how it works and why it's so essential.
Our current understanding of sleep is based largely on behavioral studies. While electroencephalogram (EEG) studies have enabled scientists to distinguish between different stages of sleep in the mammalian brain, they have not been able to define sleep at a cellular level. Now, the team of Prof. Sha Liu has expanded our understanding of this phenomenon by looking at gene expression in the brains of fruit flies.
Gene expression and glial cells
Fruit flies are more similar to humans than their appearance suggests. Of the approximately 14,000 protein-encoding genes in the fruit fly genome, about two-thirds have a human counterpart.
Single-cell genomics now allows us to look at the expression of those genes in detail – in thousands of cells! Using this technology, the team of Prof. Sha Liu has found some crucial cues in the story of sleep. They tracked changes in gene expression in the fruit fly brain and highlighted a potential role for glial cells, specific brain cells of which we know much less than neurons.
During the sleep-wake cycle of the fruit flies, the scientists observed that, gene expression in these glial cells changes with both sleep drive (or, getting tired) and the flies’ circadian rhythm (the day-night cycle of the body’s processes).
"Broadly speaking, there are two forces that determine when and how animals sleep: sleep drive, and the circadian clock. We already know these exist, but research is not clear on how these two forces interact or influence one another.", Professor Sha Liu explains. "The data we observed suggests that glial cells are an important player in integrating these two forces. We saw that glial cells contain genes that correlate with sleep drive and ones that are linked to the circadian rhythm. We hypothesize that glial cells might be integrating both pieces of information, which in turn instructs sleep behavior."

Professor Sha Liu (PI Fly Sleep Lab) and Dr. Joana Dopp (first author of study)
Sharing sleep secrets
Not only do we share a multitude of genes with the fruit fly, but we also share a common need for sleep. Sleep and the circadian clock are evolutionarily conserved, which means it is a behavior widely observed across numerous species. The molecular basis of the circadian clock was actually first discovered in the fruit fly, research which won a Nobel Prize in 2017. This, together with their small size and unparalleled genetic tricks researchers can leverage, makes these tiny creatures ideal for characterizing the mechanisms of sleep. The Liu team’s glial cell discovery now expands the scope of sleep research in this model organism.
"Our work is a massive resource for researchers interested in the mechanisms of sleep," Dr. Joana Dopp, first author of the study, explains. "We analyzed and collated a huge amount of gene expression profiles, which are freely available online to scientists studying sleep and circadian rhythms."
Through developing this resource, the team gained new insights into questions that had been puzzling the field of sleep research for some time. With the fine-grained insight new genomic technologies allow, more sleep secrets are waiting to be unearthed.
Publication
Single-cell transcriptomics reveals that glial cells integrate homeostatic and circadian processes to drive sleep–wake cycles. Dopp et al. Nature Neuroscience, 2024. DOI 10.1038/s41593-023-01549-4.
This work was funded by the European Research Council, the EOS/FWO Program, and VIB Tech Watch.
