‘Human inks’ to 3D printing human tissues: HU3DINKS project brings together expert companies for the development of human tissue-based inks
Biofabrication or 3D bioprinting aims to create tissue constructs for numerous applications including animal free testing, drug screening and regenerative medicine. One crucial aspect of the technology is the use of suitable materials to enable the 3D printing of living cells which are called bioinks. These ‘bio inks’ must support the cells and keep them alive during and after the printing process.
Currently, the majority of bioinks are derived from non-human materials (animal-based i.e., collagen or gelatin). In contrast, the present IraSME research project Human bioinks for 3D printing (HU3DINKS) aims to push this field forward by developing human tissue-based bioinks for multiple 3D bioprinting technologies.

The most commonly applied materials in 3D bioprinting are still derived from animal sources such as gelatin or collagen. Hence, there is a huge need for animal free alternatives, not only to replace animal testing, but also to resemble processes and conditions in human tissue more closely. In this respect, synthetic polymers have also been explored, and while several of these materials are safe to use in the human body, these synthetic options are an oversimplification of the complex natural situation in vivo and don’t fully bridge the gap between current in vitro tests and animal models.
The HU3DINKS project aims to overcome these limitations via the development of high-performing human tissue-based bioinks suited for different 3D bioprinting technologies including extrusion printing, and high-resolution laser-based printing. While there are already commercial human tissue-derived materials available on the market, their bioactivity and printing performance remains poor. Thus, the HU3DINKS consortium aims at converting commercially available human tissue-derived materials into bioinks, which can be printed in a straightforward manner.
Future applications of biofabrication include regeneration of human tissues and ‘organ printing’.
“However, while still many hurdles need to be overcome to convert these applications to clinical reality, the bioprinting technology already offers solutions in the field of animal free testing. Here, the ‘human 3D inks’ can make a huge difference to make bioprinting truly animal free” says Jasper Van Hoorick, CEO of BIO INX.
As a result, drugs, or cosmetics can be tested on 3D printed human tissue models, which form a better mimic of native 3D tissues in comparison to conventional 2D cell culture techniques. This approach is in line with the 3R principle* of reducing, replacing, and refining animals used for scientific purposes.
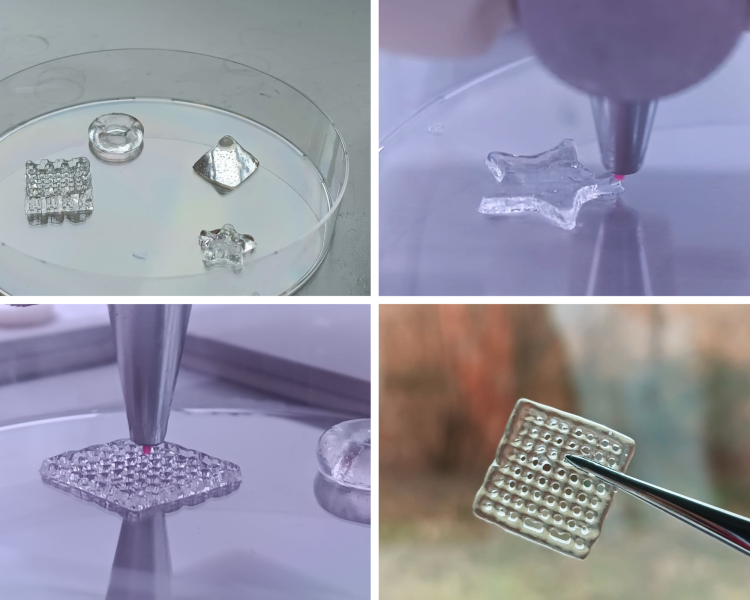
To achieve this goal, especially high-resolution bioprinting based on 2-photon polymerization (2PP) can be crucial for bringing the field to the next level, as it is the only technology allowing printing at subcellular resolution, thereby making it possible to mimic the complicated microcellular architecture. It is also one of the only technologies enabling direct printing within microfluidic chips to enable straightforward drug screening.
“The technology has made some tremendous advances in performance but is now mainly limited by the absence of high-performing biological materials. The HU3DINKS project can induce a paradigm shift in the field by truly mimicking the human cellular environment both in terms of architecture and composition.” – Markus Lunzer – Materials specialist at UpNano
The project has been granted in the framework of the international research activities by small and medium-sized enterprises (IraSME) initiative, which aims to stimulate cross-border collaborations between member countries. The HU3DINKS consortium unites partners with a unique complementary expertise from Belgium (funded by VLAIO) and Austria (funded by FFG). THT Biomaterials (Vienna, Austria) will contribute with their unique expertise on human placenta-derived materials. BIO INX (Ghent, Belgium) is expert in the field of bio ink development for multiple printing technologies and is responsible for turning the human placenta-derived materials from THT Biomaterials’ into ready-to-use printable formulations for different printing technologies. The project is also supported by Morphomed (Vienna, Austria) and their medical grade silk technology, and UpNano (Vienna, Austria), specialists in the development of 2PP (bio) high-resolution bio 3D printing technologies. Additionally, the Ludwig Boltzmann Institute for Traumatology, (the Research Center in cooperation with AUVA) will be responsible for the biological validation of the newly developed bioinks.
*3R directive 2010/63/EU
More info: https://projekte.ffg.at/projekt/4496675
