Newly discovered protein blocks sleeping sickness
Human African Trypanosomiasis (HAT), or sleeping sickness, is a neglected tropical disease that affects millions of people and is fatal if left untreated. Most treatments and preventative options, however, are not very effective. New research by the lab of Prof. Jo Van Ginderachter (VIB Center for Inflammation Research and VUB), in collaboration with Prof. Carl De Trez (VUB) and others, identifies a protein that could pave the way toward drugs against sleeping sickness. Their work appears in Nature Communications.
Sleeping sickness evades the immune system
Hitchhiking in the gut and mouthparts of the tsetse fly, microscopic parasites known as trypanosomes can cause fatal disease in mammals, including humans. In Sub-Saharan Africa, trypanosomes that infect humans cause sleeping sickness or Human African Trypanosomiasis (HAT). A similar disease in livestock is known as nagana or Animal African Trypanosomiasis (AAT).
Sleeping sickness is recognized by the World Health Organization as a neglected tropical disease, which affects millions and has high mortality rates. It is also a disease that shows a limited response to medication and for which no vaccine exists. After millions of years of evolution, trypanosomes have gained the ability to effectively escape detection by the immune system and stop potential immune responses in their tracks. How the parasites do this is still largely a mystery.
Prof. Benoit Stijlemans, first author of the study: “We know that other protozoan parasites use proteins similar to a mammalian protein called Macrophage Migration Inhibitory Factor (MIF) to manipulate the immune system of their victims. So, we went looking for a MIF equivalent in the genomes of trypanosomes that promotes sleeping sickness.”
A protein that stops trypanosomes
But a protein similar to MIF was not what the researchers found.
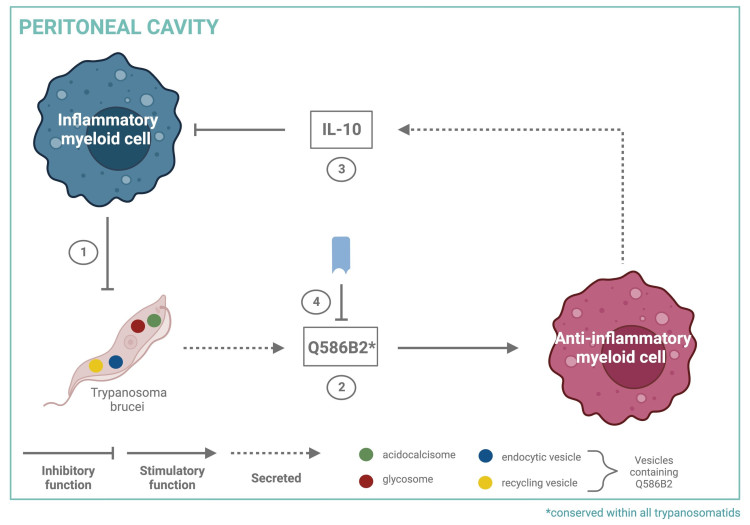
Prof. Jo Van Ginderachter, senior author of the study: “We did not find a MIF homolog in the trypanosome genome, but we did identify another protein that had interesting structural characteristics, called Q586B2.”
Deleting the gene coding for that protein from the parasite’s genome led to a lower parasite burden in the blood of infected lab mice, as well as prolonged the animals’ survival. This deletion also promoted the differentiation of the parasite into a short stumpy form, which might account for the lower parasite burden. The researchers used their new knowledge to develop two therapeutic strategies for sleeping sickness: a nanobody that blocks the action of Q586B2 designed with the help of the VIB Nanobody Core, and immunization with the protein. For both strategies, this results in a lower parasite burden and longer survival.
Prof. Jo Van Ginderachter: “The Q586B2 protein is not unique to trypanosomes. It could be the missing piece to develop a broad-spectrum intervention strategy for human diseases such as sleeping sickness, leishmaniasis, and Chagas disease as well as veterinary and economically relevant diseases such as nagana.”
A graphical summary of the findings.
Publication
Stijlemans et al. Q586B2 is a crucial virulence factor during the early stages of Trypanosoma brucei infection that is conserved amongst trypanosomatids. Nature Communications, 2024.
Funding
This work was supported by the VUB Strategic Research Program, FWO, NIH, FNRS, University of Antwerp, and KU Leuven.
