Sequana Medical announces the completion of alfapump implantations in POSEIDON, the North American pivotal alfapump study
Sequana Medical NV (the "Company" or "Sequana Medical"), an innovator in the treatment of diuretic-resistant fluid overload in liver disease, malignant ascites, and heart failure, today announces that it has completed implanting patients in POSEIDON, the North American pivotal study to support regulatory approval of the alfapump system in the U.S. and Canada, for the treatment of recurrent or refractory ascites due to liver cirrhosis.
Of the 71 patients enrolled in the Pivotal Cohort, 40 patients have been implanted with the alfapump. Pivotal patients completing the six-months post-implantation period will be included in the primary efficacy endpoint analysis, and reporting of these data is planned for Q4 2022. A further 29 patients from the Roll-In Cohort were also implanted with the alfapump and will be included in the overall safety analysis.
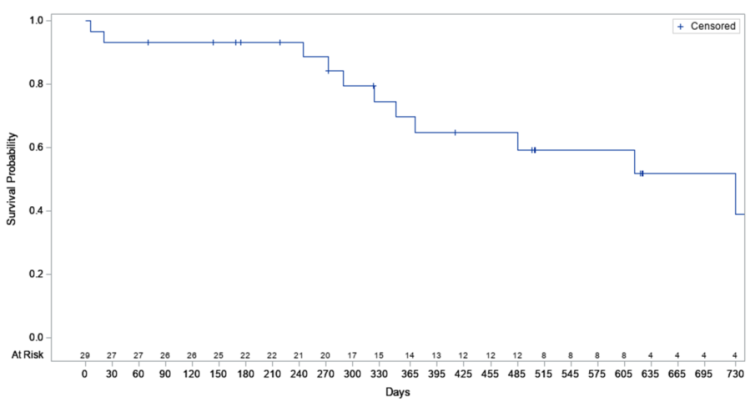
Importantly, a preliminary interim analysis1 of patient survival following alfapump implantation in the Roll-In Cohort indicated a mean survival probability of 70% at 12 months. This compares favourably with the published literature reporting a survival rate for refractory ascites patients of only 50% at 12 months. (2)
"Completing alfapump implantations is an important milestone for our POSEIDON study given the uncertainties of hospital resource availability that still exist due to COVID, and enables us to report the primary endpoint data before the end of this year as planned,” commented Ian Crosbie, Chief Executive Officer of Sequana Medical. “Given the strong efficacy results we reported in last year's interim analysis, we are confident that these implanted patients in the Pivotal Cohort will give us sufficient statistical power for the primary efficacy endpoint. For our safety evaluation, we are including the data from the Roll-In patients which will bring the total number in that assessment to approximately 70.”
“We are encouraged by our preliminary interim review of patient survival with 70% of the POSEIDON Roll-In patients alive at 12 months post-implantation,” added Dr. Gijs Klarenbeek, Senior Medical Adviser of Sequana Medical. “We believe that this survival data combined with the dramatic reduction in the rate of therapeutic paracentesis and the clinically relevant improvement in quality of life in the interim analysis of the first 26 Roll-in patients suggest that the alfapump is a highly attractive treatment option for this patient group that has been overlooked for too long.”
Kaplan Meier – Preliminary survival rate analysis of Roll-In Cohort, dated 25 March 2022
Summary of the 2nd interim analysis of Roll-In Cohort reported in July 2021
In July 2021, the Company reported the second interim analysis on 26 patients from the Roll-In Cohort, reaffirming the previous positive efficacy results of the alfapump and providing longer-term evidence of the reduction in therapeutic paracentesis and continued improvements in quality of life. Data from this Roll-In Cohort substantially exceeded the pre-defined requirements of the primary endpoints as defined for the Pivotal Cohort in the study3, demonstrating: (i) over 90% reduction in mean frequency of therapeutic paracentesis (TP) versus baseline, (ii) all patients having at least a 50% reduction in mean frequency of TP per month versus baseline, (iii) clinically important improvement in quality of life maintained even up to 12 months post-implantation, and (iv) safety profile in line with expectations.
