Scientists shed light on a sleep peptide involved in Alzheimer’s
In the early stages of Alzheimer’s disease, people exhibit cognitive stability despite the presence of toxic proteins in their brains. Researchers at the VIB-KU Leuven Center for Brain & Disease Research have uncovered early alterations in a brain region that controls neuron network activity and sleep. These findings open up exciting avenues for further research into the disease.
Most people are familiar with the devastating effects of Alzheimer's disease. However, many are not aware that the symptoms and underlying biology of Alzheimer's don't take place suddenly but instead progress over a long time.
One of the earliest stages of Alzheimer's is the prodromal phase. While the classic behavioral hallmarks of the disease may not be present during this stage, many people will experience other, more subtle differences, such as sleep disturbances. The prodromal phase is characterized by markers of the disease in the brain, such as a build-up of toxic proteins and neuron network hyperactivity.
Despite these physiological changes, cognition remains largely intact during the prodromal period of Alzheimer's disease, which strongly suggests the existence of homeostatic mechanisms that keep the brain functioning. These mechanisms are not yet well understood, although it is known that they are active during sleep and that they drive a net decrease in the strength and rate of neuron network excitation. Scientists have recently been studying the what, where, and how of this homeostatic system, the findings of which were published in Nature Neuroscience this week.
"Using a mouse model of Alzheimer's disease, we discovered that these homeostatic mechanisms are activated in the CA1 region, which is part of the hippocampus, a region of the brain important for learning and memory," Sara Calafate, one of the project leads, explains.

Joris de Wit, Sara Calafate, and Bart De Strooper
The interdisciplinary research team of neuroscientists, electrophysiologists, and pathologists – spanning labs and facilities across Belgium, Portugal, Israel, Switzerland, and the UK – found melanin-concentrating hormone (MCH) to be a key mechanism in the homeostatic system, harmonizing neuron network hyperactivity in the hippocampus.
"It was already known that neurons containing MCH reside in a small system of neurons located deep inside the brain called the lateral hypothalamic area. Despite its small size, this system is, in fact, a key player in the brain, with neurons that connect all the way to the CA1 region of the hippocampus." Joris de Wit, another co-lead based at the VIB-KU Leuven Center for Brain & Disease Research, highlights. "These MCH-expressing neurons are also known to be active during sleep and are involved in hippocampus-dependent memory. They're therefore a natural target of study for the prodromal phase of Alzheimer's. Indeed, we found that in both our Alzheimer's mouse model and in human brain tissue of Alzheimer patients, the connections of MCH neurons are damaged."
Promisingly, these scientists also found that by introducing an MCH peptide (a short string of amino acids, the building blocks of proteins) into hippocampal brain slices from an Alzheimer’s disease mouse model, they could reverse the hyperactivity in the hippocampal neuron network.
"All in all, our findings show the MCH system to be vulnerable in the early stages of Alzheimer's disease," Bart De Strooper – another study co-lead – shares. Bart De Strooper has been studying Alzheimer's at the VIB-KU Leuven Center for Brain & Disease research for over 30 years, in addition to acting as Scientific Director of the UK Dementia Research Institute. "In addition, the lateral hypothalamic area has not been previously demonstrated to be involved in the underlying neurobiology of Alzheimer's disease. This provides exciting avenues for research into the disease moving forward."
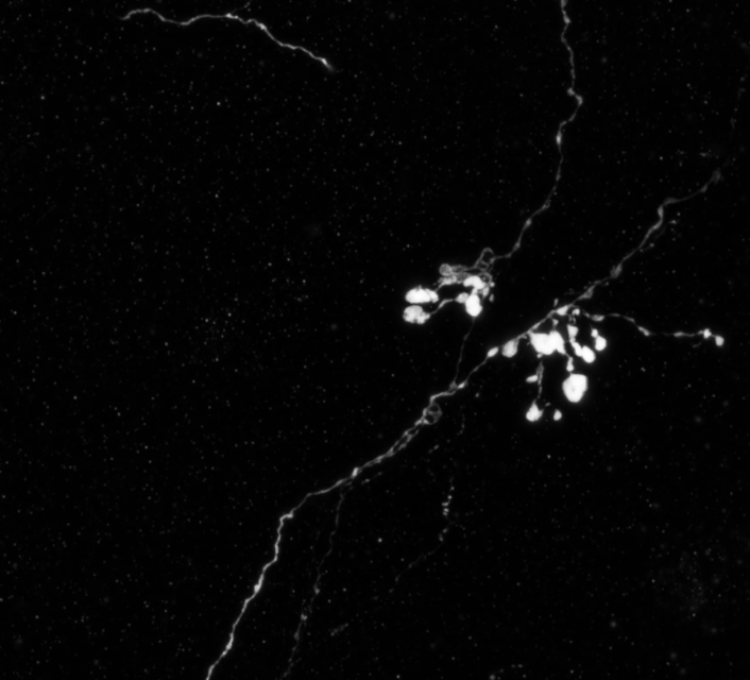
An MCH axon residing in the brain of a mouse model of Alzheimer's disease
Publication
Early alterations in the MCH system link aberrant neuronal activity and sleep disturbances in a mouse model of Alzheimer’s disease. Calafate, et al. Nature Neuroscience, 2023. https://doi.org/10.1038/s41593-023-01325-4
