Amyotrophic lateral sclerosis (ALS) is an incurable neurological disease that affects the nerves connected to our muscles. Immune abnormalities have been found in patients with the disorder, but currently little is known about the underlying disease mechanisms. Now, the Ludo Van Den Bosch group at the VIB-KU Leuven Center for Brain & Disease Research used a new coculture system that identified astrocytes as a critical player in the abnormal immune response of ALS. Their findings and model could lead to the discovery of new therapeutic strategies for the disorder. Ludo Van Den Bosch also recently won the Generet Award for his work on ALS. The paper was published in Molecular Neurodegeneration.
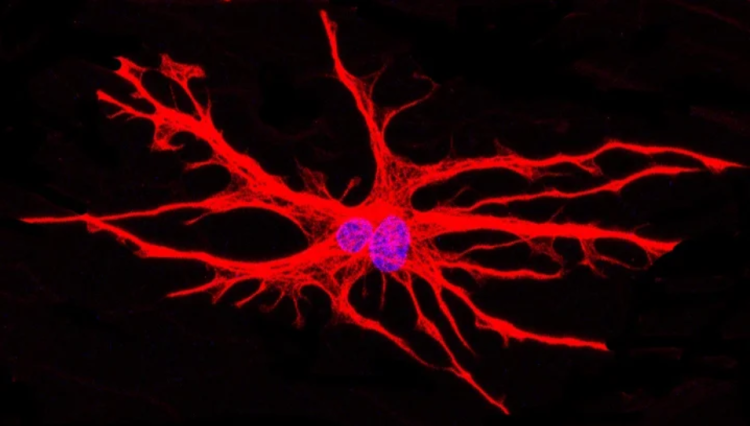
hiPSC-derived astrocytes (red) cultured in a microfluidic device. Nuclei are labeled in blue. ©Katarina Stoklund Dittlau
Ludo Van Den Bosch: “We showed that astrocytes play a central role in the disease mechanism of ALS. We also proved that our new in vitro model helps to get better insights into the processes leading to denervation and reinnervation of muscle cells and could lead to the discovery of new therapeutic strategies for incurable diseases like ALS.”
In short
ALS is an incurable, rare motor neuron disease affecting around 6 to 7 people in 100,000. Worldwide, about 100,000 people die of ALS every year.
Astrocytes are supporter cells for neurons and are known to play a role in the disease progression of ALS, but in-depth knowledge is still lacking.
With a new in vitro model using human induced pluripotent stem cells (hiPSCs), the scientists discovered that astrocytes play a central role in the abnormal immune response documented in ALS.
This discovery can provide novel avenues for therapeutic strategies, and the aim is to use the new in vitro model for drug development and testing.
ALS is the most common degenerative motor neuron disease in adults. The disorder is characterized by a selective loss of motor neurons, resulting in progressive muscle weakness and paralysis, as well as swallowing and speech difficulties. The disease can be inherited, with mutations in the FUS gene causing most young-onset cases of ALS. Patients usually succumb to the disease within 2 to 5 years after diagnosis. Every year, around 100,000 people die of ALS.
Astrocytes are star-shaped brain cells with many functions, including monitoring the electrical activity of neurons and maintaining an optimal neuronal function. They are also called ‘supporter’ cells for neurons. Astrocytes are thought to play an important role in ALS, but how exactly is not yet known. Now, the team of Ludo Van Den Bosch studied the role of astrocytes in ALS using a novel human coculture system, shedding light on their important function. This can lead to novel therapeutic possibilities for ALS.

Ludo Van Den Bosch (left) and Katarina Stoklund Dittlau (right)
Making human neuromuscular junctions in a dish
Since it is challenging to examine motor neurons in the brains and spinal cords of living ALS patients, the disease is studied in a dish. By taking skin cells from an ALS patient and temporally expressing a few genes , human induced pluripotent stem cells (hiPSCs) are generated. This is done in close collaboration with the university hospital in Leuven and Professor Philip Van Damme, neurologist at UZ Leuven. By feeding the hiPSCs with specific supplements, they can change into any cell type in the body. The lab of Ludo Van Den Bosch used hiPSCs to study the connection between motor neurons and muscle cells in microfluidic devices.
In these cultures, muscle cells are grown on one side of the dish and motor neurons on the other. The motor neurons can grow toward the muscle cells through small channels or microgrooves, where they form their specialized connections called neuromuscular junctions. This in vitro model reflects the biology of a human patient more accurately while also providing means to standardize research on neuromuscular junctions in ALS.
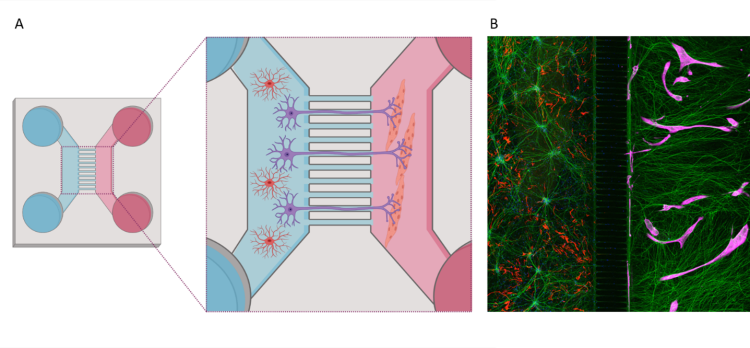
A. Schematic overview of coculture between hiPSC-derived astrocytes and motor neurons (left), and muscle cells (right), in a microfluidic device. B. Immunocytochemistry image of motor neurons (green), astrocytes (red) and muscle cells (magenta) in a microfluidic device. Schematic illustration is made with biorender.com. © Katarina Stoklund Dittlau
In this study, the researchers used hiPSC-derived astrocytes from FUS-ALS patients and incorporated these cells into the model. They saw that the growth of the neuron extensions and the formation of neuromuscular junctions were inhibited. Interestingly, the addition of healthy astrocytes rescued these impairments.
Katarina Stoklund Dittlau, first author of the study: “We saw that the astrocytes, among other functions, produced inflammatory molecules which overall inhibited the formation of the neuromuscular junctions. These data clearly demonstrate that astrocytes play a central role in the abnormal immune response documented in ALS.”
This research might provide new therapeutic pathways for ALS by targeting the astrocyte activity. The fully human multicellular microfluidics model can be used for future studies and to test therapeutic strategies that prevent denervation or stimulate the formation of neuromuscular junctions.
Publication
FUS‑ALS hiPSC‑derived astrocytes impair human motor units through both gain‑of‑toxicity and loss‑of‑support mechanisms. Stoklund Dittlau, et al. Molecular Neurodegeneration, 2023. DOI10.1186/s13024-022-00591-3.
