New protein function could explain cause of neurological disease
The nerves in your body make you move, feel, and think. CMT, or Charcot-Marie-Tooth disease, is an inherited and incurable neurological disease that occurs in one in every 2500 people. The disorder affects the nerves in your limbs, making it hard to move. More than 100 genes have been linked to CMT, but researchers remain largely in the dark about how these genes could cause the disease. Now, the lab of Albena Jordanova (VIB-UAntwerp Center for Molecular Neurology) sheds light on this. They found that a group of proteins that help build cells also have another job: making tight bundles of actin fibers in the cells. When these proteins don't work correctly, they can damage the nerves and cause the symptoms of CMT. Their work was published in the leading journal Nature Communications.
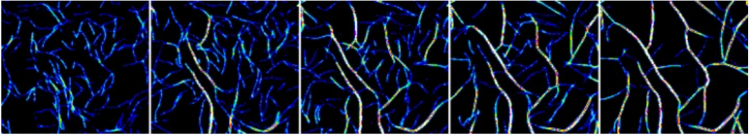
Time-lapse image showing free actin filaments (left) gradually being organized into bundles and cables by the tyrosyl-tRNA synthetase.
Albena Jordanova: "Our findings expand the current knowledge of textbook enzymes and provide a new framework on how to explain diseases like CMT. We hope that future studies will build on our research and help develop new treatments for this disease."
The most common rare disease
CMT, or Charcot-Marie-Tooth disease, is an inherited disease that affects nerve cells. Patients usually experience the first symptoms at a younger age, starting in the lower limbs, where muscle strength decreases. They also experience loss of sensation and develop skeletal deformities. CMT affects about 2.5 million people worldwide and around 3500 in Belgium. This makes it the most common rare disease.
More than 100 genes have been implicated in CMT. They are involved in various processes, making it a complicated disease to study. Previously, the group led by Albena Jordanova showed that one class of proteins, tRNA synthetases, are associated with CMT and that defects in these proteins could cause nerve damage in CMT by interfering with the gene transcription in the nucleus. However, how they cause the disease remained unknown. The Jordanova-lab now identified another disease-related function that can be attributed to synthetases and potentially explain CMT and similar neurodegenerative disorders. This discovery is a step forward toward a remedy for these diseases.

Albena Jordanova (left) and Biljana Ermanoska (right)
A new biological function
Producing proteins is one of the most essential tasks of the cells in your body, and tRNA synthetases play a significant role. These proteins ensure the correct building blocks are stacked onto a growing protein as it is made. They are constantly at work in every cell of the body. Mutations in these tRNA synthetases are associated with CMT, but strangely enough, they hardly disrupt this essential "block-building" function. This led Jordanova's team to suspect another function of these synthetases.
The researchers discovered that tRNA synthetases don't just build proteins but also make tight bundles of the actin fibers present in cells. These fibers are part of the cell's cytoskeleton, defining its shape and motility. Just like tying together multiple strands of rope creates a stronger and more stable structure, bundling actin filaments creates a larger, more robust skeleton structure that can support the cell. The problem is that defective synthetases may over-bundle actin filaments, impairing proper nerve function and causing CMT. This interference was found in both fruit flies and human cells. Excitingly, modulating the neuronal actin cytoskeleton improves the symptoms of sick flies. This supports the hypothesis that the actin cytoskeleton is involved in CMT, which may have tremendous therapeutic implications for patients.
Biljana Ermanoska, first author of the study, concludes: "Studying essential proteins comes with numerous challenges, which we tackled by adopting interdisciplinary approaches supported by the international teams of collaborators. I am excited to see future studies that will build on our findings and potentially bring together synthetases, actin dynamics, protein translation, and neurodegeneration."
Publication
Tyrosyl-tRNA synthetase has a non-canonical function in actin bundling. Ermanoska, et al. Nature Communications, 2023. DOI/10.1038/s41467-023-35908-3
