VIB research tackles the bottleneck of time-resolved Cryo-EM
Time-resolved cryo-electron microscopy (trEM) is a method to visualize how proteins function in time at near-atomic resolution. Although this method was pioneered in 1990, the potential of this technique has remained elusive because of two bottlenecks. One is the excessive consumption of proteins, and the other is that the time resolution is too low. Researchers from the lab of Prof. Rouslan Efremov of the VIB-VUB Center for Structural Biology developed a microfluidic device that tackles both issues. Their results were published in Nature Methods.
The difficulty of studying protein function
Reconstructing the exact functioning of proteins is a challenge. Protein-related phenomena like ligand binding, protein-protein interactions, and charge exchange are dynamic processes that happen in a very short time frame. This makes it difficult to study the complex conformational transitions of proteins in detail.
Time-resolved cryo-electron microscopy (trEM) is a technique that helps unravel protein mechanisms by visualizing the structure of proteins while they function. The first step of trEM is initiating the reaction by flash photolysis or mixing, followed by stopping the protein reactions at sequential time points through plunge freezing. Although trEM is a high-potential technique, the established approaches have their limits. The time resolution for plunge freezing is limited to 100 ms or the amount of protein consumed is prohibitively high, making the technique difficult to apply in practice.
The lab of Prof. Rouslan Efremov at VIB-VUB Center for Structural Biology has now developed an instrument that tackles these bottlenecks.
Elevating the trEM potential
The team developed a microfluid device that combines a fast mixer and a spray generator. It encapsulates proteins in small droplets where the reaction is initiated by rapid mixing, then it uses a laser to create tiny bubbles which help to spray the samples onto grids that can be used for imaging.
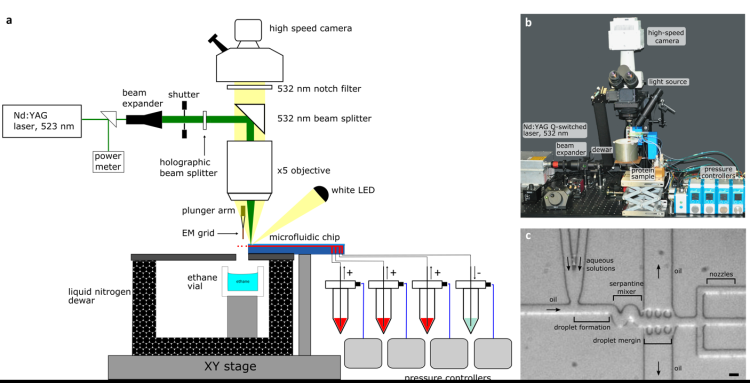
Dr. Stefania Torino, first author of the study: “The precise mixing and spraying of the tiny droplets that contain the samples tackles both the bottlenecks – the amount of protein needed and the time resolution – that are hurdles for other techniques.”
The researchers tested the device to study the dynamics of the GroEL:GroES protein complex. These proteins play an important role in helping other proteins fold properly, a highly dynamic process. With the help of their newly developed microfluid device, the team could successfully study the protein samples with a time resolution of 5 ms while consuming less than 100 nL of protein solution per EM grid. This is a massive, almost tenfold improvement over the earlier trEM technologies.
“Our new device makes trEM sample preparation potentially applicable for scarce proteins,” says Prof. Efremov. “The work does not end here. We envision that with further technical improvements to our setup, we will achieve a time resolution of below 1 ms.”
Publication
Torino et al. Time-resolved cryo-EM using a combination of droplet microfluidics with on-demand jetting. Nature Methods, 2023.
